Home
![]() Vitamin
D
Vitamin
D
Today, the vitamin D levels in man are measured in clinical laboratories on a
routine basis. "Routine" means, that in many cases the laboratory staff is not
well-trained, and has to do a lot of work.
Unfortunately, the assay procedure for the measurement of vitamin D is rather
complicated, and involves several crucial steps, as described below.
Assay procedure version: July 2000
|
Vitamin D ELISA for the quantitative
determination of |
This assay procedure is also available as single-page lab protocol, in the format MS WORD 97. Click here for download.
Vitamin D is a secosteroid hormone involved in the intestinal absorption of calcium and in the regulation of calcium homeostasis. There are two different forms of vitamin D, namely D3 and D2, which are structurally very similar. The latter one is a synthetic product, which is predominantly absorbed via fortified food. Physiological Vitamin D3 levels result from dietary uptake but also from biosynthesis in the skin from 7-dehydrocholesterol and UV-light due to sun exposure. The vitamin is then hydroxylated in the liver to 25-hydroxyvitamin D (25-OH Vit D) which is the major circulating metabolite of Vitamin D. Although the biological active form of vitamin D is 1,25(OH)2 Vit D, synthesised in the kidney, it is widely accepted that the measurement of circulating 25-OH Vit D provides better information with respect to the patients vitamin D status and is used for diagnosis of hypovitaminosis (1,2). The concentration of 25-OH Vit D decreases with age and deficiency is common among the elderly (3,4).
Clinical aspects:
Clinical applications of 25-OH Vit D measurements are the diagnosis and therapy control of postmenopausal osteoporosis (5,6), rickets, osteomalacia, renal osteodystrophy, pregnancy, neonatal hypocalcemia and hypoparathyroidism. In addition, a prevalence of subclinical vitamin D deficiency in different European countries has been discussed lately (7). Vitamin D intoxication mostly occurs from large intake of pharmaceutical preparations of vitamin D and may lead to hypercalcemia, hypercalcuria and nephrocalcinosis in susceptible infants (8).
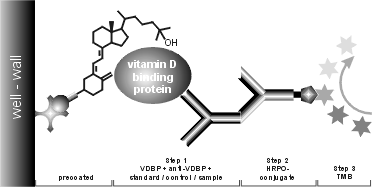
This test kit is a competitive binding protein assay for the measurement of 25-OH Vit D. It is based on the competition of 25-OH Vit D present in the sample with biotinylated 25-OH Vit D (tracer) for the binding pocket of vitamin D binding protein (VDBP, Gc-globulin). Since all circulating 25-OH Vit D is bound to VDBP in vivo, samples have to be precipitated with organic solvents to extract the analyte. The supernatant is used without further treatment in the test.
In the first step, vitamin D binding protein, anti-vitamin D binding protein
antibody and samples/standards/controls are added. 25-OH Vit D present in the
sample then competes with the 25-OH-Vit D-Biotin, bound to the well, for the
specific binding sites of the binding protein. Hence, with increasing
concentrations of 25-OH Vit D in the sample the amount of binding protein
immobilised to the well via the tracer is reduced. Simultaneous addition of an
antibody specific for this protein yields a complex, which is finally
quantitated by incubation with a host specific peroxidase labelled antibody
using TMB as enzyme substrate. The amount of colour developed is inversely
proportional to the amount of 25-OH Vit D present in the
standards/samples/controls (i.e. the less vitamin D that is present in the
sample, the higher the intensity of the colour developed).
A standard curve is plotted and the concentrations of 25-OH Vit D in the samples
are calculated from this curve.
Below, the assay scheme is shown graphically:
Serum- or plasma samples must be centrifuged within one hour. Store samples at -20 °C if not assayed within 24 hours. Lipemic samples may give erroneous results and must be centrifuged for 10 min. at 13000 rcf (rotational centrifugal force = g) to seperate the lipids from the plasma. Samples should be mixed well before assaying. We recommend duplicates for all tests.
Reagent preparation:
Assay procedure:
Handle tubes carefully after centrifugation! The supernatant must be perfectly clear and well separated from the pellet at the bottom of the tubes. The supernatant contains the extracted 25-OH Vit D and should be assayed immediately. If not possible, keep at 4°C for max. 1 hour.
If no reference wavelength is available, read only at 450 nm. If the extinction of the zero standard exceeds the measurement range of the photometer, absorption must be measured immediately at 405 nm against ³ 620 nm as reference.
Sample / Standard / Control preparation:
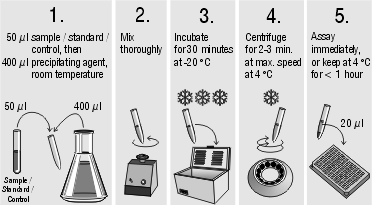
Assay procedure:
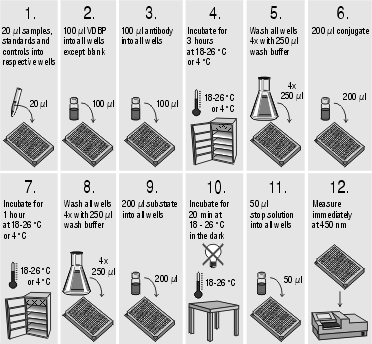
A calibration curve is constructed from the standards. Commercially available software can be used as well as graph paper. Results of the samples are read from this calibration curve.
THE CALIBRATION CURVE IS NOT LINEAR, therefore a spline- or 4PL algorithm is recommended.
Standard range:
2.5 - 100 ng/ml
1 ng/ml = 2.5 nmol/l
1 nmol/l = 0.4 ng/ml
A typical standard curve looks like this:
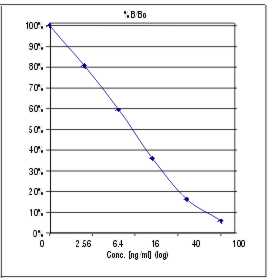
Detection limit:
The detection limit is the concentration of 25-OH Vit D at 95% B/B0.
For this assay the detection limit was determined as 0.6 ng/ml.
Cross reactivity:
| 24,25-(OH)2 vitamin D3 25-OH vitamin D2 1,25-(OH)2 vitamin D3 vitamin D2 & D3 |
100% 100% 1% 1% |
Sample volume / type:
50 µl of human plasma or serum. EDTA or Heparin plasma are the preferred
matrices for this assay to compare results to other 25-OH-D analysis systems.
Precision:
Intraassay:
4 samples were tested in 16 replicates. Two repeats of the experiments are
shown.
|
Sample |
Run 1 (n=16) |
Run 2 (n=16) |
||
|
Mean (ng/ml) |
CV |
Mean (ng/ml) |
CV |
|
|
1 |
15.5 |
8 % |
11.5 |
10 % |
|
2 |
43.6 |
9 % |
35.4 |
11 % |
|
3 |
5.8 |
17 % |
6.6 |
14 % |
|
4 |
22.5 |
9 % |
19.3 |
9 % |
Interassay:
6 patient samples were tested in duplicates (3 runs).
|
Sample Nr: |
Run 1 |
Run 2 |
Run 3 |
Mean (ng/ml) |
CV |
|
1 |
9.3 |
8.2 |
8.6 |
8.7 |
6.6 % |
|
2 |
16.2 |
20.8 |
17.3 |
18.1 |
13.3 % |
|
3 |
35.1 |
43.2 |
36.5 |
38.3 |
11.3 % |
|
4 |
49.2 |
50.1 |
43.3 |
47.5 |
7.8 % |
|
5 |
32.4 |
38.2 |
38.2 |
36.3 |
9.2 % |
|
6 |
8.2 |
10.8 |
10.7 |
9.9 |
15.0 % |
Recovery:
10 plasma samples were spiked with 62.4 ng/ml 25-OH Vit D - plasma standard and
measured in the assay.
|
Spike |
Measured |
Recovery |
|
62.4 ng/ml |
58.7 ng/ml |
94 % |
Incubation times:
3 h / 60 min / 20 min
Storage: 4 °C
Shelf life: 6 months from day of production
General:
All test components of human source were tested with 3rd generation tests against HIV-Ab and HBsAG; all components were found to be negative. However, they should be handled and disposed as if they were infectious, since no test method can offer complete assurance.
Last modified: 19. Sep 04